Early vision for next-gen implants
Company founded and research into a new generation of breast implants begins with a focus on women's safety and long-term outcomes.
Choose Your Motiva Experience
Discover Motiva Ergonomix® implants, learn how breast augmentation works, and connect with certified surgeons across Indonesia.

Access product specifications, clinical evidence, training programs, and premium partnership support for your practice.


We’re proud to share that Motiva Implants® have been awarded the prestigious Allure Best of Beauty Breakthrough Award!
With thousands of products evaluated every year and only a select few chosen, Motiva® is the first and only plastic surgical and breast implant brand ever to receive this award.
Important Safety Information for Patients: Motiva Ergonomix® Breast Implants are indicated for breast augmentation for women of at least 22 years old. Breast augmentation includes primary breast surgery to increase the breast size, as well as revision surgery to correct or improve the result of an original primary breast augmentation surgery (i.e., revision-augmentation). Breast Implant surgery is contraindicated in women with active infection anywhere in their bodies, with existing cancer or pre-cancer of their breast who have not received sufficient treatment for those conditions, or who are currently pregnant or nursing. Adequate studies have not been performed to confirm the safety of breast implant surgery in women with these conditions or under these circumstances; therefore, if you have any of the conditions or circumstances listed above, breast augmentation surgery with implants should not be performed at this time. Failure to take into consideration these contraindications may increase the risks involved with breast implant surgery and have the potential to cause harm. Patients should be advised that key complications have historically been associated with silicone gel breast surgery and implantation of silicone gel breast implants including, but not limited to, capsular contracture, implant removal, reoperation, infection, and rupture. Further, breast implants are not lifetime devices and patients should visit their healthcare professional, as recommended.Physician Safety Statement: The Motiva SmoothSilk® Round and SmoothSilk Ergonomix® Silicone Gel Breast Implants are indicated for breast augmentation for women of at least 22 years old. Breast augmentation includes primary breast surgery to increase the breast size, as well as revision surgery to correct or improve the result of an original primary breast augmentation surgery (i.e., revision-augmentation). Breast Implant surgery is contraindicated in women with active infection anywhere in their bodies, with existing cancer or pre-cancer of their breast who have not received sufficient treatment for those conditions, or who are currently pregnant or nursing. Prior to use, plastic surgeons should review all risk information with women who are considering breast implant surgery. This risk information can be found in the Directions for Use which is provided with each device and found on www.motivausa.com. Plastic surgeons should advise patients of the key complications that have been historically associated with breast implant surgery and implantation of silicone gel breast implants, including, but not limited to, capsular contracture, implant removal, reoperation, infection, and rupture. Plastic surgeons should also advise patients that breast implants are not lifetime devices and patients should follow-up with them, as recommended.Sale and Distribution: The sale and distribution of this device is restricted to users and/or user facilities that provide information to patients about the risks and benefits of this device in the form and manner specified in the approved labeling provided by Establishment Labs®.References1. Establishment Labs® Holdings Corp. Establishment Labs® Notes Presentation of 5-Year Results from Motiva® U.S. IDE Study at the Aesthetics MEET 2025. https://investors.establishmentlabs.com/press-releases/press-releases-details/2025/Establishment-Labs-Notes-Presentation-of-5-Year-Results-from-Motiva-U-S--IDE-Study-at-The-Aesthetic-MEET-2025/default.aspx. Published March 20, 2025. Accessed March 20, 2025.
2. Establishment Labs®, Post-Market Surveillance Preliminary Results Q4 2024. Internal Data on File. [This includes breast implants and breast tissue expanders worldwide.]
Motiva Ergonomix® is designed to move, feel, and behave like natural breast tissue — giving women in Indonesia a more harmonious, natural-looking result.
Motiva® is trusted by women and surgeons in more than 80 countries.Our mission is simple: elevate safety, advance innovation, and support aesthetic results that feel beautifully natural.Motiva implants are known for:
Thoughtful engineering
Exceptional safety track record
Aesthetic outcomes that blend seamlessly with your body

Soft, natural feel
Dynamic movement that mimics natural tissue
SilkSurface™ technology for gentler healing
Lower inflammation and capsular contracture rates
BluSeal® safety barrier for added protection
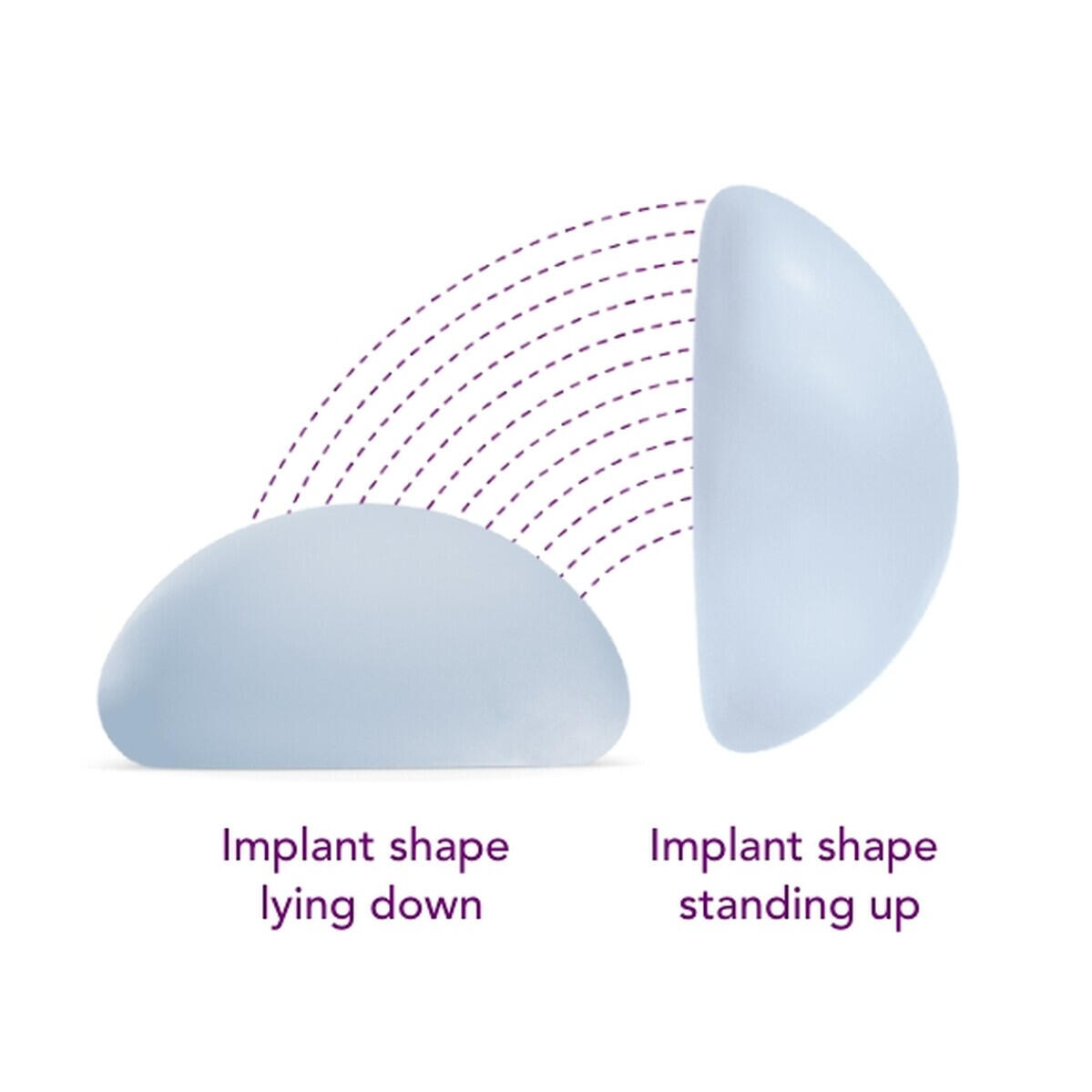
Motiva Ergonomix® contains an advanced SmoothSilk®/SilkSurface™ shell and ProgressiveGel ULTIMA™ — engineered to move the way natural breasts do.Whether you're resting, moving, or exercising, Ergonomix® implants adapt smoothly and naturally.

Standing

Lying Down
Placement
Look

Feel
From placement to daily movement to aesthetic outcome, the implant adapts seamlessly, giving you a breast shape that feels harmonious, confident, and uniquely yours.Tailored for women in Indonesia who want results that look natural and feel like part of their own body.
See how Motiva Ergonomix® enhances soft, natural silhouettes while preserving each woman’s unique shape.
Your peace of mind matters.
Motiva implants are engineered with multiple layers of safety and transparency:

We want to support you every step of the way as a patient and give you the utmost confidence in choosing Motiva implants.
First-Year Free Coverage
5Y Motiva® Program
Registering your implants and taking advantage of one our warranties is a sign that you are serious about your decision and value your health.

All Motiva® Implants are covered by the Always Confident Warranty® against rupture for the lifetime of the device and by our Product Replacement Policy in the event of capsular contracture (Baker grades III and IV) for a period of 10 years.
To extend the coverage of your Motiva® breast implants, Motiva® offers an Extended Warranty Program that applies to Motiva® breast implants through an additional fee to be covered by or on behalf of the patient during the first 90 days after the initial breast surgery as specified below.

First-Year Free Coverage

For first year free coverage, patients must register their Motiva® breast implants with Qid® through the motiva.health website or the MotivaImagine® app within the first 90 days after the initial breast surgery.**Subject to additional Terms & Conditions
5Y Motiva® Program

The 5-year extended warranty program applies to all Motiva® breast implants with Qid® that have been registered in the MotivaImagine® website or app when a non-refundable fee has been applied to enroll the patient in the program. Patients must register the implanted devices within the first 90 days after the initial breast surgery.
Additional Info

In addition to the replacement product, these programs will support you with financial assistance applicable to the cost of one revision surgery in the event of a warranty claim for rupture or capsular contracture (Baker Grades III and IV).Motiva® Implants silicone breast implants warranty shall not apply to any implantations performed without strict accordance to current product “Directions for Use, Sterile Silicone Breast Implants Motiva Implant Matrix” and accepted surgical procedures by appropriately qualified and licensed surgeons. Motiva® Implants standard and extended warranty programs do not apply to cases of:
Revision surgery patients with previous history of capsular contracture with other brands of breast implants different than Motiva®
Removal of intact breast implants for capsular contracture grades I or II
Removal of intact breast implants for size alteration
Removal of intact breast implants due to wrinkling or rippling
Loss of shell integrity caused by or during reoperative procedures
Loss of shell integrity resulting from open capsulotomy or closed compression capsulotomy procedures
Loss of shell integrity resulting from sharp instrument damage
Registering your implants is for your own peace of mind and years of confidence in your decision. You have a 90-day window after surgery to do it. We’ll walk through it with you!

Implant registration must be done by the patient
This process is solely applicable to Motiva® Implants
They must be registered within 90 days of your surgery to be valid
It has no cost
To purchase an extended warranty, your Motiva® Implants must be correctly registered first
Sign-up or Sign-in to the MotivaImagine® App

Open the MotivaImagine® website and sign up with either your Facebook or Google account, or with your email address. Fill your Personal Information as requested.
Review and Accept the Terms & Conditions

Read and accept the Terms & Conditions by checking the boxes you agree to and click “Submit”.
Register Your Implants

From the Home page click “Register my implants”, select your registration method (with SN or ESN), then complete the implant and surgery information required in each step.
Registration Confirmation

You will receive an e-mail confirming that your implant(s) have been registered successfully.
Disclaimer
*The information provided is for informational and educational purposes only, the content herein is not intended as a substitute for consultation with a physician. Motiva Ergonomix® implants have received Premarket Approval (PMA) from the Food and Drug Administration (FDA) for Augmentation Indication, and are commercially available in the US.**The statements and opinions presented here are applicable to each individual. Results will vary and may not be representative of the experience of others. All statements are voluntarily provided and are not paid, nor were they provided with free products, services, or any benefits in exchange for said statements. The statements are representative of patient experience; the exact results and experience will be unique and individual to each patient.
Natural dynamics has been a key missing factor in traditional implants due to their predefined shape, which remains unchanged whether the patient is prone, supine, or upright.Motiva® incorporates this characteristic into Ergonomix® through the unique viscoelastic properties of its proprietary Ultima® gel – holding a round shape when the patient is lying down and forming a natural teardrop shape when she is standing.
Ergonomix® implants adjust to movement in real time. Patients experience a soft, organic feel that blends with their natural anatomy.
The implant’s flexibility and shell behavior support the use of smaller incisions—benefitting both aesthetic outcomes and recovery.
Disclaimer
*This information is intended solely for the use of healthcare professionals. A healthcare professional must always rely on his or her own professional clinical judgment when deciding whether to use a particular product when treating a particular patient. Motiva® does not dispense medical advice and recommends that healthcare professionals be trained in the use of any particular product before using it in a procedure or surgery. A healthcare professional must always refer to the package insert, product label and/or instructions for use before using any Motiva® product. The information presented is intended to demonstrate particular products, as well as the breadth of Motiva® product offerings. Products may not be available in all markets because product availability is subject to the regulatory and/or medical practices in individual markets. Please contact your local representative if you have questions about the availability of specific products in your area. Motiva® products that are CE marked are marked according to the applicable EU Regulations and Directives.
Motiva Ergonomix® combines natural aesthetics, reduced complication rates, and evidence-based safety — helping surgeons deliver results their patients trust.
The global shift toward soft, natural-looking results has made Motiva one of the fastest-growing implant brands worldwide.
Key advantages for surgeons:
Nanotextured SilkSurface™ for reduced inflammation
ProgressiveGel Ultima™ for natural dynamics
BluSeal® safety barrier for rupture protection
Lower rates of capsular contracture
Strong published clinical evidence
Streamlined warranty and after-sales process

A proprietary multi-layer system unifying the implant’s structure and allowing it to bend and squeeze with Motiva MinimalScar®.
“The blue implant.” The only device of its kind with a visual safety barrier, achieving the lowest level of diffusion of tested commercially available implants.

A gel with a unique set of rheological properties that provides a better adaptation to change than other silicone gels tested, resembling the movement of natural tissue.

A controlled advanced smooth surface that improves biocompatibility.
All the information you should know about the implants, kept safely through a battery-free and passive radiofrequency.

We’re proud to share that Motiva Implants® have been awarded the prestigious Allure Best of Beauty Breakthrough Award — a recognition that celebrates innovation, safety, and meaningful impact in the beauty industry.
With thousands of products evaluated ever year and only a select few chosen, Motiva® is the first and only plastic surgical and breast implant brand ever to receive this award.

SmoothSilk® is a revolutionary biocompatible* 4-micron surface produced by the mandrel imprinting technique and designed to optimize the body’s immune response.This proprietary surface is scientifically shown to promote:
low inflammatory response,
low friction,
low bacterial attachment,
resulting in soft breasts, low capsular contracture rates, and no reported primary device-related proliferative diseases (BIA-ALCL, BIA-SCC and B-cell lymphomas).
Motiva Implants® are 100% filled with the latest, 6th generation gel that is both highly cohesive and viscoelastic.This gel composition is designed to result in:
Less folds
Less friction
Lower risk of rupture
With unique rheological properties: low viscosity and high elasticity. This enables enhanced adaptability and responsiveness, resulting in soft consistency and gravitational shifting.
Where beauty and safety are seamlessly integrated. Designed to fit every woman’s unique goals, our implants offer safety features that you and your patients can trust.Ready to learn more?

Crafted with leading surgeons to combine innovation and patient satisfaction. Whether you want to enhance your patient’s natural beauty or restore their confidence, our tailored approaches make every surgery a masterpiece.Blend our innovation with your expertise.

(Kaplan-Meier, KM), including MRI cohort
| Primary augmentation | 2-year (N=451) 95%CI | 3-year (N=451) 95%Cl | 4-year (N=451) 95%Cl | 5-year (N=451) 95%Cl |
|---|---|---|---|---|
| Capsular contracture (Baker Grade III/IV) | 0.5% | 0.5% | 0.5% | 0.5% |
| Rupture, suspected or confirmed (MRI Cohort) | 0.6% | 0.6% | 0.6% | 0.6% |
| Breast pain | 0.5% | 0.7% | 0.9% | 1.2% |
| Infection | 0.9% | 0.9% | 0.9% | 0.9% |
| Implant removal, with or without replacement | 1.6% | 1.6% | 1.8% | 3.1% |
| Any reoperation | 5.7% | 6.1% | 6.8% | 8.8% |
| Any complication | 7.5% | 8.4% | 9.6% | 12.0% |
All data presented is preliminary 2, 3, 4 and 5-year follow up IDE Study data and does not reflect the final study results nor establish the ultimate safety or effectiveness of the device.
*Based on the 5-point Likert Scale. The office-visit follow-up rate at 3-years follow-up was of 92.7%.
References
1. MRI cohort, N=176
2. Any surgery on the breast or chest area, device or non-device related, including size change
3. Any device or non-device related event, including reoperation
Boost patient conversion and satisfaction and expand your expertise by offering Motiva® implants through Seffyre, the exclusive Motiva® agent in Indonesia.
Disclaimer
*This information is intended solely for the use of healthcare professionals. A healthcare professional must always rely on his or her own professional clinical judgment when deciding whether to use a particular product when treating a particular patient. Motiva® does not dispense medical advice and recommends that healthcare professionals be trained in the use of any particular product before using it in a procedure or surgery. A healthcare professional must always refer to the package insert, product label and/or instructions for use before using any Motiva® product. The information presented is intended to demonstrate particular products, as well as the breadth of Motiva® product offerings. Products may not be available in all markets because product availability is subject to the regulatory and/or medical practices in individual markets. Please contact your local representative if you have questions about the availability of specific products in your area. Motiva® products that are CE marked are marked according to the applicable EU Regulations and Directives.
Motiva® combines innovative products and tools with the most advanced surgical approaches to create designed surgeries for custom patient outcomes.


With its unique combination of tools and techniques, the Motiva MinimalScar® procedure enables surgeons to insert Motiva® Implants using incisions as small as 2.5 cm – around half the size of the current industry standard.Motiva® Implants are uniquely flexible and durable enough to be inserted through smaller incisions, thanks to their rheological properties and surface technology relative to other traditional implants.

Innovative tools and less invasive techniques leave patients with minimal scarring by reducing the required incision size to almost half to the standard size.

The MotivaImagine® Insertion Sleeve, with a smaller opening to fit through a smaller incision.
MotivaImagine® Ultralight LED Retractor, which is narrower than typical retractors and comes with a built-in LED light to increase visibility through a smaller incision.

Motiva Ergonomix® durability and flexibility.

Designed to mimic the look, feel, and movement of natural breast tissue.
Boost patient conversion and satisfaction and expand your expertise by offering Motiva® implants through Seffyre, the exclusive Motiva® agent in Indonesia.
Disclaimer
*This information is intended solely for the use of healthcare professionals. A healthcare professional must always rely on his or her own professional clinical judgment when deciding whether to use a particular product when treating a particular patient. Motiva® does not dispense medical advice and recommends that healthcare professionals be trained in the use of any particular product before using it in a procedure or surgery. A healthcare professional must always refer to the package insert, product label and/or instructions for use before using any Motiva® product. The information presented is intended to demonstrate particular products, as well as the breadth of Motiva® product offerings. Products may not be available in all markets because product availability is subject to the regulatory and/or medical practices in individual markets. Please contact your local representative if you have questions about the availability of specific products in your area. Motiva® products that are CE marked are marked according to the applicable EU Regulations and Directives.
Motiva® by Establishment Labs® is a first-of-its-kind medical technology company focused on breast aesthetics and breast reconstruction*, bringing Femtech innovations to the market for women’s health and well-being.

Establishment Labs® devices** in worldwide market since 2010.
where Establishment Labs® devices** have been approved.
Establishment Labs® actively supports research.
Applications approved/pending in 21 jurisdictions.
**This includes breast implants and breast tissue expanders worldwide.

More than two decades ago, Motiva® by Establishment Labs® changed the industry paradigm by putting women’s health first. With innovation, safety, and technology, we started a revolution in the Femtech sphere by creating a new space that cared for women’s unmet needs within the breast aesthetic and reconstruction fields.The main goal: to create Femtech solutions that heal, normalize, and democratize breast health and aesthetics.
The Heart of Creation is our hub for innovation in Costa Rica, bringing together women’s health contributors, patients, healthcare professionals, and partners. Inspired by the Sulàyöm mountain’s creation tale, our campus includes the Global Learning Center, an amphitheater for medical community growth, an in-house content studio, and Research, Development, and Innovation labs.

1. IntroductionThis Privacy & Cookies Notice explains how PT Seffyre Pure Aesthetic (“Seffyre”, “we”) collects, uses, stores, and protects your personal data when you visit:Indonesian-language site: https://motivaindonesia.co.idEnglish-language site: https://en.motivaindonesia.co.idThese sites provide educational information about Motiva Ergonomix breast implants and breast augmentation surgery for patients and plastic surgeons in Indonesia. No online purchases or contracts are formed through these sites.The sites are intended for Indonesian residents aged 21 years and above. By using the sites, you are deemed to have read and understood this Notice.2. Who We Are & How to Contact UsData controller:
PT Seffyre Pure Aesthetic
Based in South Jakarta, IndonesiaContact for all privacy-related and general inquiries:Email: [email protected]Business WhatsApp: +62 81888888888We have not appointed a formal Data Protection Officer (DPO) at this time. Privacy matters are handled by our team, which you can reach using the email address above.3. Scope of This NoticeThis Notice applies to all processing of personal data we carry out in connection with your use of the sites and any communications arising from the sites, including:Site usage data (e.g., via Google Analytics); andCommunication data you send to us via WhatsApp buttons on the sites.This Notice complements other terms on the sites (such as the Terms of Use & Medical Disclaimer) and does not replace medical advice from your own doctor.4. Sources & Types of Personal Data We Process
4.1. No Online FormsWe do not provide:Online consultation forms,User account registration, orAny file upload features on the sites.If you choose to contact us, you do so via WhatsApp, not via web forms.4.2. Site Usage Data (Google Analytics)When you visit our sites, we use Google Analytics to automatically collect certain usage information. This may include:IP address (which may be shortened/anonymized depending on configuration),Browser type and version,Device type and operating system,Pages visited, dates and times of visits, visit duration,Referring URL,General interactions with content (e.g., clicks on certain buttons).We primarily use this information in aggregate statistical form, not to personally identify you, unless we are required to do so by law.4.3. Communication Data via WhatsAppIf you click the WhatsApp button on the sites, you will be redirected to the WhatsApp application (or WhatsApp Web) and communicate with us through our business WhatsApp account.In this context, we may process:The phone number you use,Your display name on WhatsApp,The content of your messages, voice notes, photos, or other communications you send,Any other information you voluntarily share (for example, the clinic where you work, your city of residence, aesthetic concerns, or suggested consultation times).Important note:Personal medical decisions and detailed medical history must be discussed directly with your doctor, not solely through the sites or via WhatsApp.Please avoid sending highly sensitive medical information via WhatsApp unless strictly necessary. If you nevertheless choose to share such information, we will treat it as sensitive personal data and seek to protect it in accordance with applicable law, but we do not control the WhatsApp platform itself.4.4. Data You Voluntarily ProvideWe may also process other personal data you voluntarily provide in your communications with us, such as:Full name,Clinic or hospital name,Position and specialization (for example, plastic surgeon),Preferences regarding products or education,Your availability or schedule for being contacted.5. Purposes & Legal Bases for ProcessingWe only process your personal data as far as necessary for legitimate purposes and in line with Indonesian Personal Data Protection Law and related health regulations. In summary:5.1. Providing General & Educational InformationTo present educational content about Motiva Ergonomix and breast augmentation-related topics.To ensure the sites are accessible and relevant for visitors in Indonesia.Legal basis:Our legitimate interests in educating the Indonesian market and promoting our services in a responsible way.5.2. Responding to Patient & Doctor InquiriesTo read and respond to messages you send via WhatsApp, such as requests for further information, introductions to clinics, or general product-related questions.To maintain records of communications for reasonable follow-up and legitimate business relationships.Legal basis:Your own request as part of taking steps toward a legitimate service or business relationship,Our legitimate interests in providing good-quality responses to prospective patients and doctors.5.3. Analysing Site Usage & Improving Our ServicesTo understand how visitors interact with the sites (e.g., which pages are most frequently viewed).To improve the structure, security, and relevance of site content.Legal basis:Your consent to the use of analytics cookies (Google Analytics) given via the cookie banner/settings on the sites;Our legitimate interests in improving the quality of information and user experience, to the extent permitted by law and your cookie choices.5.4. Compliance with Legal Obligations & Protection of RightsTo comply with legal and regulatory obligations (such as reporting, record-keeping, or tax requirements).To establish, exercise, or defend legal claims.To respond to lawful requests from regulators or government authorities.Legal basis:Applicable legal obligations,Our legitimate interests in protecting our rights, privacy, security, and assets, as well as those of others.6. Data Retention PeriodsWe retain your personal data only for as long as necessary for the purposes described above, unless a longer period is required or permitted by law.WhatsApp communication dataRetained for up to five (5) years from the date of our last interaction with you,Or longer if needed for legal, accounting, or dispute-resolution purposes.Google Analytics dataRetained for up to 26 months from the date of collection, orFor the default retention window set by Google Analytics, whichever is shorter.After these periods, your data will be deleted, anonymised, or aggregated so that it no longer identifies you.7. Data Recipients & International TransfersWe do not sell your personal data. However, we may share your personal data with the following categories of third parties, where necessary:IT and hosting providersProviding infrastructure, site hosting, system maintenance, and security services.Cloud and email service providersSuch as business email, cloud storage, and collaboration tools.Google AnalyticsAs our web analytics provider. Google may process data in multiple countries.We configure the service to comply with applicable laws and to minimise privacy risks.WhatsApp (Meta Platforms)The messaging platform you use to contact us.WhatsApp has its own privacy policy and terms of use which we do not control.Professional advisorsSuch as auditors, legal counsel, or accounting firms, where necessary.Government authorities or regulatorsWhere required by law or by a valid order.Cross-border transfers:
Your personal data may be stored and/or processed outside Indonesia (for example, in data centres used by global cloud providers). We will take reasonable steps to ensure that such data receives a level of protection materially consistent with applicable standards in Indonesia, including through appropriate contractual arrangements and technical/organisational safeguards.8. Cookies & Tracking Technologies
8.1. What Are Cookies?Cookies are small text files stored on your device when you visit a website. They help the site remember your preferences and understand how the site is used.8.2. Types of Cookies We UseEssential cookiesNecessary for the basic functioning of the sites (for example, properly displaying pages, providing basic security).Without these cookies, the sites may not function correctly.Essential cookies do not require separate consent, but you may still block them via your browser settings.Analytics cookies (Google Analytics)Help us understand how visitors use the sites (which pages are viewed, how long visits last, etc.).The information is used in aggregate form to improve the content and structure of the sites.We use analytics cookies only with your consent, which you can give or refuse through the cookie banner/settings on the sites.8.3. Managing CookiesYou have several options:Manage your cookie preferences through the cookie banner/settings that appear when you first visit the sites.Change your browser settings to refuse all cookies or to alert you when a cookie is being sent.Delete cookies already stored on your device via your browser’s settings menu.Please note that if you block certain cookies (especially essential cookies), parts of the sites may not function properly.9. Data SecurityWe implement reasonable technical, administrative, and organisational measures to protect your personal data from unauthorised access, use, disclosure, alteration, or destruction.However, no system or data transmission over the internet is completely secure. We encourage you to:Use secure devices and connections, andAvoid sending highly sensitive medical information through channels where it is not necessary, including WhatsApp.10. Your Rights as a Data SubjectUnder applicable Indonesian personal data protection laws, you may have the following rights (subject to certain conditions and limitations):Right to information – to be informed about how we process your personal data.Right of access – to request confirmation as to whether we process your data and to obtain a copy of certain data.Right to rectification – to request that inaccurate or incomplete data be corrected or updated.Right to deletion – to request deletion of certain data where it is no longer needed or where processing is unlawful.Right to withdraw consent – where processing is based on your consent (for example, analytics cookies), you may withdraw that consent at any time without affecting the lawfulness of prior processing.Right to object – to object to certain processing based on our legitimate interests, on grounds relating to your particular situation.Right to lodge a complaint – with the competent personal data protection authority in Indonesia if you believe your rights have been violated.You can exercise these rights by contacting us at: [email protected]
.
We will aim to respond within a reasonable period and in accordance with any deadlines set by law.11. Use of the Sites by Individuals Under 21The sites are intended for users aged 21 years and above.
We do not knowingly collect personal data from individuals under 21. If we become aware that such data has been collected without appropriate authorisation, we will take reasonable steps to delete it.12. Governing Law & Dispute ResolutionThis Notice is governed by and interpreted in accordance with the laws of the Republic of Indonesia.Any dispute arising in connection with this Notice or our processing of your personal data may be brought before the courts in South Jakarta, Indonesia, without prejudice to your rights under applicable law to bring matters before other competent authorities or forums.13. Changes to This NoticeFrom time to time, we may update this Privacy & Cookies Notice to reflect:Changes in our data processing practices,Changes in legal or regulatory requirements, orAdjustments to the services we provide through the sites.If we make material changes, we will notify you by updating this Notice on the sites and revising the “Last updated” date below. The version in force is the latest version available on the sites.Last updated: 3 December 2025
1. Introduction1.1 These Terms of Use & Medical Disclaimer (“Terms”) govern your access to and use of the Motiva Indonesia website:Indonesian site: https://motivaindonesia.co.idEnglish site: https://en.motivaindonesia.co.id(together, the “Site”).1.2 The Site is operated by PT Seffyre Pure Aesthetic, a company incorporated under the laws of the Republic of Indonesia (“Seffyre”, “we”, “us”).1.3 By accessing or using the Site, you represent and warrant that you:(a) are at least 21 years old; and(b) have read, understood, and agree to be bound by these Terms.If you do not agree to these Terms, please do not access or use the Site.2. About Motiva Indonesia and Scope of the Site2.1 The Motiva Indonesia Site is an educational website providing general information about:Motiva Ergonomix® breast implants; andbreast augmentation procedures and related topics,
intended for residents of Indonesia, including prospective patients and plastic surgeons/clinics in Indonesia.2.2 There are no online sales or booking of services/products through the Site. All implant purchases and medical treatment arrangements occur offline via professional channels and the respective healthcare institutions.2.3 Information on the Site may be changed, updated, or removed by Seffyre at any time without prior notice.3. Legal Relationship and Medical Responsibility3.1 The Site provides general information only about Motiva Ergonomix® and breast health–related topics. Such information:is non-personalised (it does not take into account your individual condition); andis not a substitute for professional medical advice, diagnosis, or treatment.3.2 By using the Site, you understand and agree that:no doctor–patient relationship is created between you and Seffyre;no medical consultation, diagnosis, or prescription services are provided through the Site; andany medical decision must only be made after direct consultation with a qualified plastic surgeon or other licensed healthcare professional.3.3 In emergencies or potentially life-threatening situations, you must immediately contact local emergency services or visit the nearest healthcare facility, and must not rely on the Site for assistance.4. Audience and Territory4.1 The Site is primarily intended for residents of Indonesia aged 21 and above, including prospective patients and healthcare professionals (plastic surgeons, clinics, hospitals).4.2 The Site may be accessible from other jurisdictions, but we:do not represent that the content of the Site complies with laws or regulations outside Indonesia; anddo not guarantee that the products/procedures mentioned on the Site are available or appropriate outside Indonesia.5. Intellectual Property Rights5.1 Unless stated otherwise, all:text, images, videos, graphics, illustrations, and other content on the Site; andthe structure, look, and arrangement (layout) of the Site,
are owned or controlled by PT Seffyre Pure Aesthetic and are protected by copyright, trademark, and other intellectual property laws in force in Indonesia.5.2 Motiva® and all related trademarks, logos, and product names are the property of their respective rights holder(s). PT Seffyre Pure Aesthetic is an authorised distributor of Motiva in Indonesia, but does not own the Motiva® brand itself.5.3 Without prior written consent from Seffyre and/or the relevant rights holder (as applicable), you must not:copy, distribute, modify, publish, upload, publicly display, or reproduce any part of the Site content for commercial or competing purposes;use any trademarks, logos, or branding elements of Seffyre or Motiva in any manner that may cause confusion or imply a relationship or endorsement that does not exist;create derivative works based on content from the Site.5.4 You may:view and print copies of the Site content for personal reference and/or non-commercial professional reference (for example, for discussion with your doctor), provided that such use:does not alter the content; andincludes appropriate attribution.6. Permitted and Prohibited Use6.1 You may only use the Site for purposes that are lawful and consistent with these Terms.6.2 You must not:use the Site for any unlawful, fraudulent, or harmful purpose;infringe the intellectual property rights, privacy rights, or other rights of Seffyre, the Motiva rights holder, healthcare professionals, patients, or any other party;upload or disseminate any material that is abusive, threatening, defamatory, obscene, discriminatory, or otherwise objectionable;attempt to gain unauthorised access to any part of the Site’s technical infrastructure (including servers, databases, or other systems);engage in any activity that may disrupt, damage, or disable the Site (including through malware, DDoS attacks, or exploitation of security vulnerabilities);carry out large-scale automated data extraction (such as by crawler, robot, spider, or mass scraping) without Seffyre’s prior written consent;use the Site in any way that may damage the reputation of Seffyre, Motiva, or healthcare professionals mentioned on the Site.6.3 We may, at our sole discretion:monitor compliance with these Terms to the extent permitted by law; andrestrict, suspend, or terminate your access to the Site if we believe that you have breached these Terms or applicable laws.7. Medical Disclaimer for Patients7.1 General Information Only, Not Personal Medical Advice
All medical information on the Site:is provided for general educational purposes only regarding Motiva breast implants and breast augmentation procedures;does not take into account your health status, medical history, aesthetic goals, or specific needs; andis not intended to replace direct consultation with a plastic surgeon or other healthcare professional.7.2 Medical Decisions Must Be Made by a Doctor Who Has Examined You
Only a qualified plastic surgeon who has examined you in person can:assess whether a particular implant or procedure is appropriate for you;explain the risks, benefits, contraindications, and potential side effects;advise on the appropriate size, profile, and type of implant; andexplain the expected results and limitations.7.3 Do Not Make Medical Decisions Based Solely on the Site
You must not:decide to undergo or refuse any medical procedure solely on the basis of the information provided on the Site; ordelay, disregard, or discontinue any medical care or consultation you are receiving or intend to receive solely because of information read on the Site.Always consult a doctor before making any medical decision.7.4 Not All Procedures Are Suitable for Everyone
Each individual has different anatomy, breast tissue characteristics, medical history, and aesthetic preferences. Therefore:not all procedures or implants mentioned on the Site will be suitable or safe for every person; andany results shown (for example, before–after photos) are illustrative and do not guarantee similar results for you.7.5 Medical Emergencies
In case of an emergency or if you experience serious symptoms (such as severe pain, shortness of breath, high fever, extreme swelling, or signs of serious complications):immediately contact local emergency services; orpromptly go to the nearest healthcare facility.The Site does not provide emergency services or real-time medical advice.7.6 Surgeon or Clinic Listings on the Site
If the Site includes:listings of plastic surgeons, clinics, or hospitals; orlanguage such as “partner clinics”, “trusted surgeons”, or similar terms,you understand that:such surgeons and clinics are independent healthcare providers who are solely responsible for their own medical acts and omissions;Seffyre does not control their clinical decisions, diagnoses, surgical procedures, or medical services; andSeffyre does not guarantee clinical outcomes, success rates, or your satisfaction with the services provided by such surgeons/clinics.Any claims, complaints, or disputes relating to medical treatment must be directed to the relevant doctor or healthcare facility.8. General Disclaimer and Limitation of Liability8.1 “As Is” and “As Available”
The Site and all its content are provided “as is” and “as available”, without any warranties of any kind, whether express or implied.8.2 No Warranties
To the fullest extent permitted by applicable law, Seffyre does not warrant that:the information on the Site is completely complete, accurate, up-to-date, or error-free;the Site will always be available without interruption, or free from viruses, malware, or other harmful components;the Site will be fully compatible with your device, operating system, or browser; orthe results obtained from using the information on the Site will meet your expectations or specific objectives.8.3 Limitation of Liability
To the fullest extent permitted by the laws of the Republic of Indonesia, Seffyre and its directors, employees, and affiliates shall not be liable for:any indirect, incidental, special, consequential, or punitive damages;loss of profit, loss of business opportunity, loss of data, or business interruption;
arising out of or in connection with:your use of or inability to use the Site;your reliance on information contained on the Site; orany actions or decisions you take based on information from the Site.8.4 Maximum Aggregate Liability
If, notwithstanding the above, Seffyre is found liable to you by a court of competent jurisdiction, then Seffyre’s total aggregate liability to you for all claims relating to the Site shall be limited to the maximum amount permitted under the laws of the Republic of Indonesia.9. Third-Party Links9.1 The Site may contain links to websites operated by third parties (such as clinic websites, doctor websites, professional associations, or other manufacturers). These links are provided for your convenience only.9.2 Seffyre:does not control and is not responsible for the content, security, or privacy practices of such third-party websites; anddoes not endorse, guarantee, or recommend the information, products, or services offered through such third-party websites, unless expressly stated.9.3 You access third-party websites linked from the Site at your own risk.10. Changes to the Site and These Terms10.1 Seffyre may at any time:update, modify, or remove content on the Site;change the appearance, structure, or technical features of the Site; and/orsuspend or discontinue (in whole or in part) access to the Site,
with or without prior notice.10.2 Seffyre may also update these Terms from time to time. The latest version will:be published on the Site; andtake effect from the “Last updated” date stated at the bottom of these Terms.10.3 By continuing to access or use the Site after changes are made, you are deemed to have accepted the updated Terms.11. Termination11.1 Seffyre may, at any time and without liability to you:restrict, suspend, or terminate your access to the Site if there is a suspected breach of these Terms or of applicable laws; and/orremove or block the use of particular devices or IP addresses from accessing the Site.11.2 Provisions which by their nature are intended to survive termination (including, without limitation, intellectual property rights, limitations of liability, and governing law) shall continue in full force and effect.12. Governing Law and Jurisdiction12.1 These Terms are governed by and construed in accordance with the laws of the Republic of Indonesia.12.2 Any dispute arising out of or in connection with the Site or these Terms, to the extent not resolved amicably, shall be submitted to the courts in South Jakarta, Indonesia.13. Language13.1 These Terms are drawn up in Indonesian and English. Both versions are intended to have the same meaning and legal effect.13.2 In the event of any inconsistency or difference in interpretation between the Indonesian and English versions, the Indonesian version shall prevail and be binding.14. Contact UsIf you have any questions about these Terms or the Site, you may contact us at:Email: [email protected]Business WhatsApp: +62 81888888888Last updated: 3 December 2025








